Commission Published Guidance on Medical Devices Labeling Requirements
The Commission and the EU Member States have created MDR and IVDR tables with an overview of language requirements for manufacturers of medical devices.
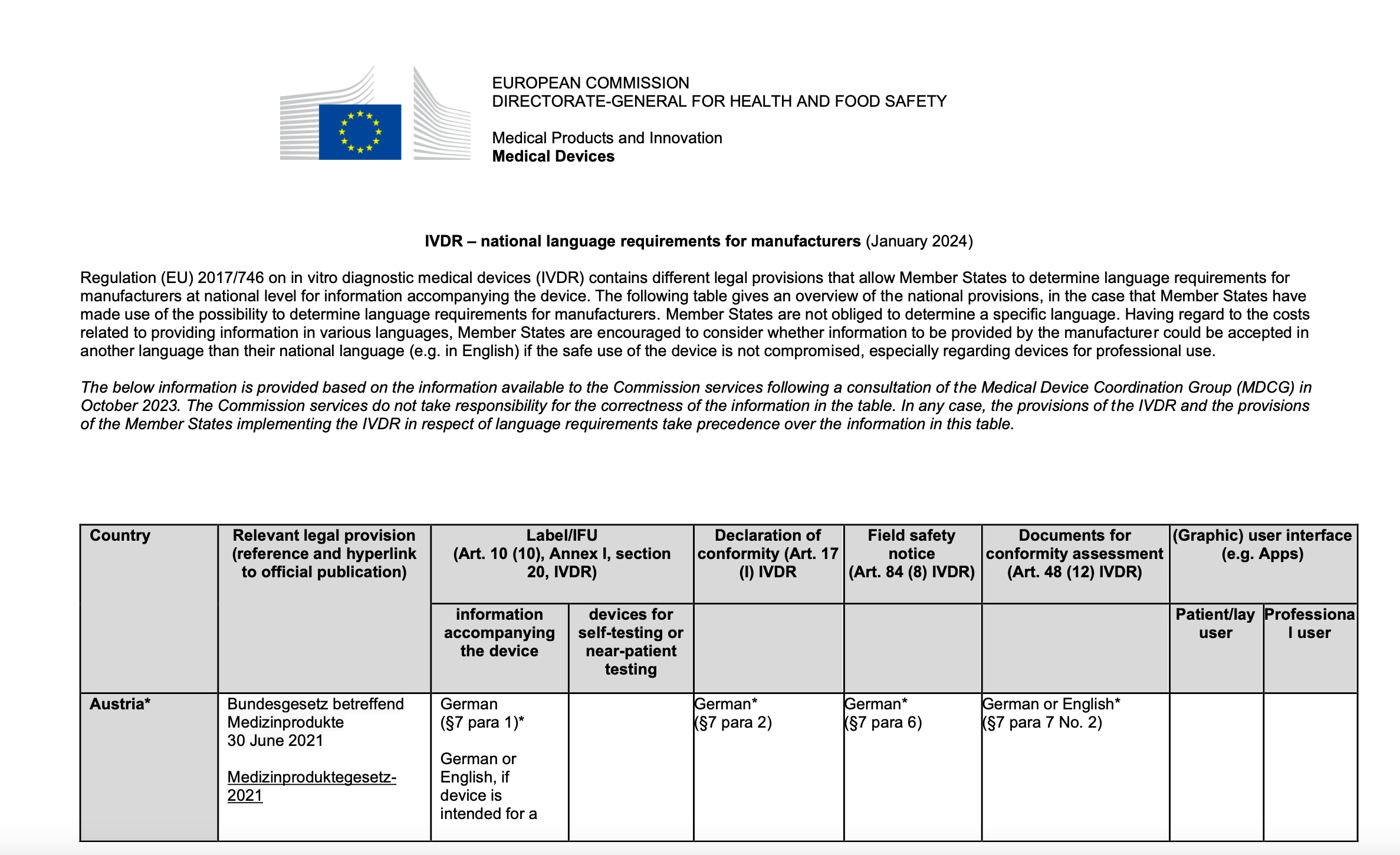
The Commission and the EU Member States have created MDR and IVDR tables with an overview of language requirements for manufacturers of medical devices.